Click to see full Important Safety Information & Prescribing Information
What is LUCEMYRA?
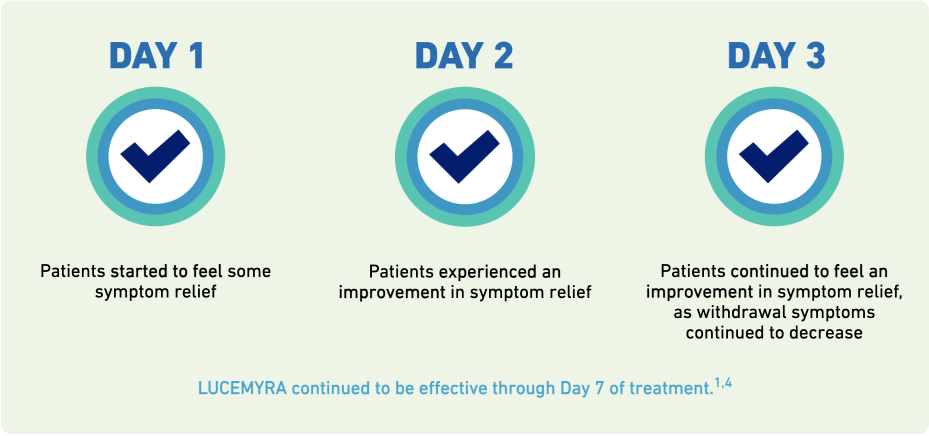
LUCEMYRA is a non-opioid prescription medicine used in adults to help with the symptoms of opioid withdrawal that may happen when you stop taking an opioid suddenly.
LUCEMYRA will not completely prevent the symptoms of opioid withdrawal and is not a treatment for opioid use disorder.
Important Safety Information
LUCEMYRA can cause serious side effects, including low blood pressure, slow heart rate, and fainting. Watch for symptoms of low blood pressure or heart rate, including dizziness, lightheadedness, or feeling faint at rest or when quickly standing up; if you experience these symptoms, call your healthcare provider right away and do not take your next dose of LUCEMYRA until you have talked to your healthcare provider. Avoid becoming dehydrated or overheated and be careful not to stand up too suddenly from lying or sitting, as these may increase your risk of low blood pressure and fainting.
When your treatment is complete, you will need to stop taking LUCEMYRA gradually, or your blood pressure could increase.
After a period of not using opioid drugs, you can become more sensitive to the effects of opioids if you start using them again. This may increase your risk of overdose and death.
Before taking LUCEMYRA, tell your healthcare provider about all your medical conditions, including if you have low blood pressure, slow heart rate, any heart problems including history of heart attack or a condition called long QT syndrome, liver or kidney problems, or if you drink alcohol. Tell your healthcare provider if you are pregnant, plan on becoming pregnant, or are breastfeeding; it is not known if LUCEMYRA can harm your unborn baby or whether LUCEMYRA passes into your breast milk.
Especially tell your healthcare provider if you take benzodiazepines, barbiturates, tranquilizers, or sleeping pills, as taking these with LUCEMYRA can cause serious side effects.
The most common side effects of LUCEMYRA include low blood pressure or symptoms of low blood pressure such as lightheadedness, slow heart rate, dizziness, sleepiness, and dry mouth.
To report SUSPECTED ADVERSE REACTIONS or product complaints, contact US WorldMeds at 1-833-LUCEMYRA. You may also report SUSPECTED ADVERSE REACTIONS to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Click here to see full Prescribing Information.